September 16 commemorates the date of the signing of the Montreal Protocol, critical for preservation of the Ozone Layer. Facts about ozone, different layers of the Earth’s atmosphere, effects of ozone depletion, Kigali Agreement and more — here’s all that you must know.
The sky’s ‘safeguard’ or the Earth’s ‘sunscreen’ that protects us from too much ultraviolet radiation from the sun, needs protection itself. What is the problem and what is the world doing to keep the ozone layer strong? Let’s learn…
September 16 is designated as the International Day for the Preservation of the Ozone Layer by the United Nations to protect the fragile shield of the Ozone layer, which protects the planet Earth from the harmful ultraviolet radiations from the Sun. India has been celebrating this day since 1995. The theme for this year is “Montreal Protocol: Advancing Climate Actions”.
First thing first, let’s know about ‘Ozone’
Ozone (O3) is a reactive gas, consisting of three oxygen atoms which can be natural or man-made and found in the Earth’s high atmosphere (stratosphere). The word ‘ozone hole’ refers to areas or regions harmed by damaging UV radiations.
The ozone present in the stratosphere, at a height of around 15 to 30 km is produced naturally by the interaction of solar ultraviolet light with molecular oxygen (O2). On the other hand the tropospheric or ground-level ozone is principally produced by photochemical processes that involve volatile organic compounds (VOC) and nitrogen oxides.

(Text source: http://www.noaa.gov)

So how is ozone layer depleted
The ozone layer is depleted in both hemispheres of the Earth, specifically Antarctica in the Southern Hemisphere and the Arctic in the Northern Hemisphere. However, the phenomenon is more recognised in Antarctica than in the Arctic.
The mechanism of the ozone hole is intimately related to the temperature of the stratosphere. If the temperature goes below -78 degrees Celsius, stratospheric clouds form, worsening the status of the ozone hole.
The extent of the ozone hole above Antarctica varies each year, beginning in August and closing in November or December.
Some consumer goods and industrial activities release “halogen source gases” into the atmosphere. The ozone layer is weakened by these gases bringing chlorine and bromine into the stratosphere. For instance, practically all air conditioning and refrigeration systems contain chlorofluorocarbons, eventually making their way to the stratosphere where they break down to produce chlorine atoms that deplete the ozone layer.
Halons, used in fire extinguishers contain ozone-depleting bromine atoms. Here comes the important role of the Montreal Protocol — governing the global production and consumption of all primary halogen source gases resulting from human activity.
Above all, why is ozone depletion harmful
Exposure to UltraViolet B (UVB) rays has various effects on:
A. Human Health: The depletion of the ozone layer has resulted in low amounts of ozone, which means reduced protection from the sun’s rays and increased exposure to UVB radiation at the Earth’s surface. Laboratory and epidemiological research show that UVB induces non-melanoma skin cancer and plays an important role in malignant melanoma formation. UVB has also been related to the development of cataracts, which are cloudy lenses in the eyes.
B. Flora Life: UVB radiation affects the plants — both physiologicaly and in their development processes. The growth of trees is affected directly and indirectly by the UVB radiation. For instance, nutrient distribution and developmental phase are indirectly affected.
C. Marine Life: Phytoplankton production is limited to the euphotic zone, the upper layer of the water column that receives enough sunshine to support net productivity. Solar UVB radiation has been proven to influence phytoplankton orientation and motility, resulting in lower survival rates. Small increases in UVB radiation can cause population declines in small marine organisms, having repercussions for the entire marine food chain.
D. Terrestrial Life: Increased UVB radiation can have an impact on terrestrial biogeochemical cycles, affecting both sources and sinks of greenhouse gases and chemically significant trace gases such as carbon dioxide and carbon monoxide.
By the way, do you know what the Montreal Protocol is exactly
Adopted on September 16, 1987, the Montreal Protocol is the landmark multilateral environmental agreement that regulates the production and consumption of man-made chemicals referred to as ozone-depleting substances (ODS). The stratospheric ozone layer is damaged by these ODS.
According to the UNEP website,
the Montreal Protocol phases down the consumption and production of the different ODS step-wise, with different timetables for developed and developing countries. Under the treaty, all parties have specific responsibilities related to the phase-out of the various groups of ODS, control of ODS trade, annual reporting of data, national licensing systems to check the imports and exports of ODS and other matters.
There are different responsibilities for developing and developed countries, but the countries have binding, time-targeted, and measurable commitments.
Implementation of the Montreal Protocol
Hydrochlorofluorocarbons (HCFCs) are used for different purposes such as refrigeration, air conditioning, and foam applications. However, they were phased out under the Montreal Protocol due to their ozone layer depleting effects. These are considered ODS and powerful greenhouse gases. The most commonly found HCFC has a global warming potential (GWP) around 2,000 times that of CO2. The parties agreed in 2007 to phase out HCFCs to maintain the climate of the Earth.
Universal ratification
On September 16, 2009, the Vienna Convention and the Montreal Protocol became the first treaties in United Nations history to be universally ratified.
Do you know: The Vienna Convention was the first treaty of any kind to be signed by all participating countries, taking effect in 1988 and being universally ratified in 2009.
The Kigali Amendment
Hydrofluorocarbons (HFCs) are a distinct family of compounds that were developed to help with the timely phase-out of CFCs and HCFCs as non-ozone-depleting alternatives. Nowadays, HFCs are often found in refrigerators, air conditioners, foams, aerosols, and other products. These substances are safe for the stratosphere’s ozone layer, however, they have high global warming potentials (GWPs) of around 12000-14,000.
The Parties to the Montreal Protocol voted to phase down HFCs at their 28th Meeting of the Parties, held on October 15, 2016, in Kigali, Rwanda. HFCs were added to the list of compounds under international control, and a plan to gradually reduce them by 80-85% by the late 2040s was approved.
Wondering how much the situation has improved now
Since 2000, the condition of the ozone hole has been improving due to the effective implementation of the Montreal Protocol.
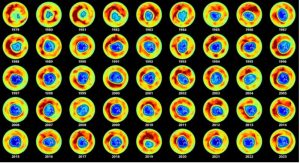
(Image credit: European Environmental Agency)
According to a recent scientific assessment, the effective implementation of the Montreal Protocol. will help the ozone layer to restore to 1980 levels over Antarctica by 2066, the Arctic by 2045, and the rest of the world by 2040.
Eliminating ozone-depleting chemicals also has significant climate change benefits. These compounds are also potent greenhouse gases, with some being hundreds or thousands of times more harmful than carbon dioxide, the most abundant greenhouse gas and primary cause of global warming. The research said that global compliance with the Montreal Protocol will assure the prevention of 0.5 to 1 degree Celsius of warming by 2050.
SOURCE: ![]()
To view the original article, click here.

